In the Single Market, independent distributors of medicines can buy pharmaceuticals in any EU or EEA country and under strictly regulated conditions transport, repackage and resell them in another. This activity is called parallel export and import of medicines, and it is possible because of differences in medicine prices between different countries of the EU and EEA. Independent distribution is the only form of competition in branded medicines.
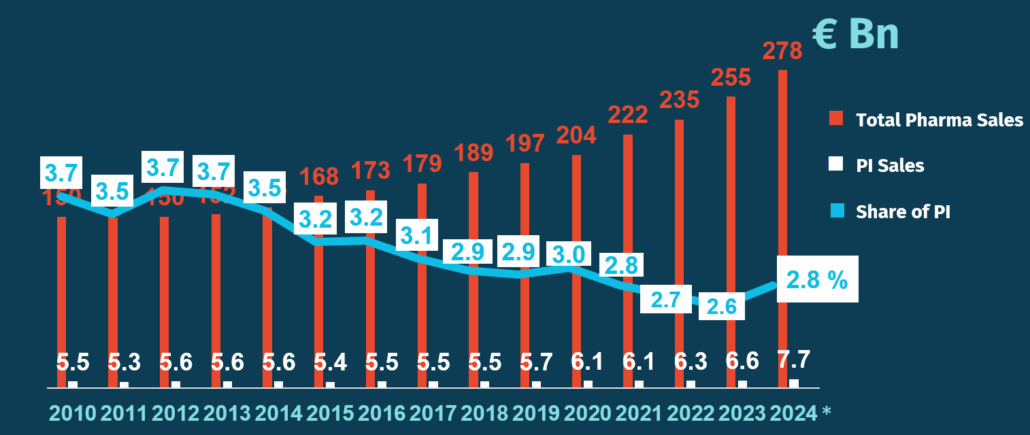
While the medicines sales in the EU have been rapidly growing over the last decade, reaching €255 billion in 2023, the turnover of EU parallel imports has remained stable in the same period at around €6-7 billion. In 2024, the sales of parallel imports represented about 2.8%, €7.67 billion of the total sales of the pharmaceutical market in Europe.
Parallel imports of medicines originate from different European countries and, contrary to common belief, most of the parallel imported medicines are sourced in high-income countries instead of low-income countries. In fact, more than 50% of the parallel imports are sourced in high income countries, and the distribution of the trade flows is balanced between southern, northern, eastern and western countries of Europe. These are the main findings of a study based on extensive data provided by the members of Affordable Medicines Europe in 17 countries, that represent around 85% of the parallel import sales in the continent. You can read more about it from our 2023 Trade Flow study.
When a new medicine is developed, the manufacturer receives patents and trademarks to protect its innovation. However, under the EU principle of exhaustion, once a medicine has been lawfully placed on the market anywhere in the EU/EEA, the manufacturer can no longer use its patent or trademark to prevent that product from being further distributed in another Member State.
Medicine prices vary significantly across Europe. This is because manufacturers set different prices in each market and because every country has its own rules for pricing and reimbursement. As a result, the same medicine can be cheaper in one high-income country than in another, and not necessarily in markets traditionally seen as “low price.”
Within this landscape, licensed wholesalers who have lawfully purchased medicines from the manufacturer may resell part of their stock to other EU/EEA markets once national demand has been met. This redistribution takes place entirely within the EU’s regulatory framework and ensures the free movement of goods in the Single Market.
Independent distributors operate within this framework. They legally purchase medicines in one EU/EEA country where there is excess supply and, under very strict regulatory conditions, redistribute them to another where there is a need, at a lower price than the standard local rates offered by the originator, creating savings in the destination market. Trade in medicines flows in all directions across the Single Market. Despite perceptions that parallel imports come mainly from lower-priced countries, the price of a specific medicine can in fact be much lower in a traditionally high-priced market than in an averagely low-priced one. Indeed, more than 50% of all imported medicines originate from high-income countries.
Parallel importers and exporters are true entrepreneurs. Majority of them are small and medium-sized enterprises. They are registered as pharmaceutical wholesalers who supply the intra-European market (exporters) or who place medicines from another country’s market in a destination market (importers) who must in addition hold a manufacturing authorisation for the necessary repackaging of the medicines. The contribution of independent distributors to innovation in the pharmaceutical sector lies in their ability to identify and procure products with relevant price differences and thus foster competition and provide a service to healthcare systems and patients by making these medicines more affordable and accessible. Independent distributors must meticulously research, calculate and meet the costs associated with purchasing, transport, warehousing, insurance, repackaging, quality assurance, regulatory compliance, distribution and marketing of products.
Parallel distribution is regulated under the Directive on Medicines for Human Use (Directive 2001/83/EC), the Falsified Medicines Directive (Directive 2011/62/EU), the Trade Marks Directive (Directive 2015/2436/EU), the Regulation for the authorisation and supervision of medicinal products for human and veterinary use and establishing a European Medicines Agency (Regulation (EC) No 726/2004), the Commission communication on the Community marketing authorisation procedures for medicinal products (Commission Communication 98/C 229/03) and national legislation.
Different requirements befit the two types of distributors, parallel exporters and importers, in the pharmaceutical market.
Exporters must possess:
Importers must possess:
The legal basis of independent distribution of medicines lays in the principles of free movement of goods and exhaustion of intellectual property (IP) rights within the EU Single Market. Independent distribution is a strictly regulated sector under EU and national legislation.
The legality has been recognized by the Court of Justice of the European Communities since the late 1960s and 1970s. ((For patents; 29 February 1968, in Case C-24/67, Parke Davis and Co. v Probel (principle) and 31 October 1974, in case C-15/74, Centrafarm BV et Adriaan de Peijper v Sterling Drug Inc. For Trademarks; 13 July 1966, in joint cases C-56/64 and C-58/64, Établissement Consten S.à.R.L. and Grundig-Verkaufs-GmbH v Commission; 31 October 1974, in case C-16/74, Centrafarm BV et Adriaan de Peijper v Winthrop BV; and 23 May 1978, in case C-102/77, Hoffmann-La Roche et Co. AG v Centrafarm Vertriebsgesellschaft Pharmazeutischer Erzeugnisse MBH. On Trademarks the legality was confirmed also under the Trademark Directive on 16 July 1998, in case C-355/96, Silhouette International Schmied GmbH v Hartlauer Handelgesellschaft GmbH.)).
Independent distribution of medicines within the Single Market is based on Articles 34 and 35 of the Treaty on the Functioning of the European Union (TFEU), subject to the derogations regarding the protection of human health and life and the protection of industrial and commercial property, provided by Article 36 of the TFEU.
Parallel distribution of medicines in the EU’s Single Market is furthermore protected by the principle of exhaustion of intellectual property rights ((See Trademarks Directive (Directive 2015/2436/EU), and jurisprudence from the CJEU. For case law on patents; 29 February 1968, in Case C-24/67, Parke Davis and Co. v Probel (principle) and 31 October 1974, in case C-15/74, Centrafarm BV et Adriaan de Peijper v Sterling Drug Inc. For Trademarks; 13 July 1966, in joint cases C-56/64 and C-58/64, Établissement Consten S.à.R.L. and Grundig-Verkaufs-GmbH v Commission; 31 October 1974, in case C-16/74, Centrafarm BV et Adriaan de Peijper v Winthrop BV; and 23 May 1978, in case C-102/77, Hoffmann-La Roche et Co. AG v Centrafarm Vertriebsgesellschaft Pharmazeutischer Erzeugnisse MBH. On Trademark the legality was confirmed also under the Trademark Directive on 16 July 1998, in C-355/96, Silhouette International Schmied GmbH v Hartlauer Handelgesellschaft GmbH.)).
The exhaustion principle prevents IP right-owners from restricting further distribution of their products once they have placed these on a given EEA market themselves. That is because the IP owners have already extracted their ‘ownership profit’ with the first sale in the Single Market. This right cannot be used to obtain a double profit from IP.